> เกี่ยวกับ SICRES
SICRES : Siriraj Institute of Clinical Research
Your Success Is Our Success
News & Events

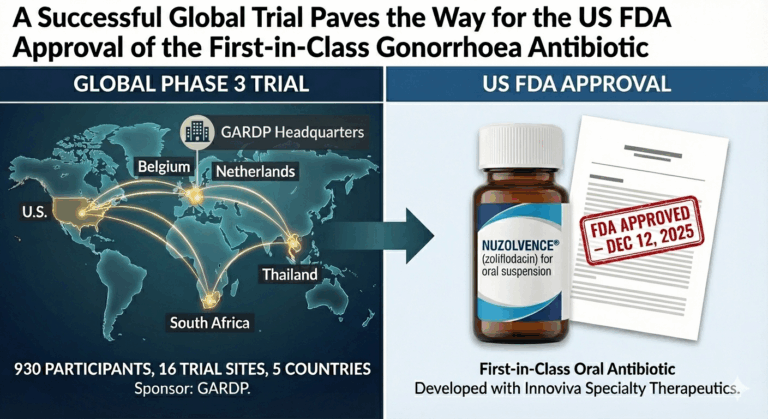
A Successful Global Trial Paves the Way for the US FDA Approval of the First-in-Class Gonorrhoea Antibiotic
A major milestone in global health has been achieved with the U.S. FDA approval of NUZOLVENCE® (zoliflodacin), the first new antibiotic developed exclusively for the treatment of gonorrhoea in decades.

Announcement of Eligible Participants for the Clinical Vaccine Trial Training Program!
ประกาศรายชื่อ ผู้มีสิทธิ์เข้าอบรม “หลักสูตรการวิจัยวัคซีนทางคลินิก สำหรับแพทย์ เภสัชกรณ์ และพยาบาลวิจัย” Clinical Vaccine Trial Expert Program!

น้อมส่งเสด็จสู่สวรรคาลัย
น้อมส่งเสด็จสู่สวรรคาลัย
สมเด็จพระนางเจ้าสิริกิติ์ พระบรมราชินีนาถ พระบรมราชชนนีพันปีหลวง
สถิตในดวงใจตราบนิรันดร์
ด้วยเกล้าด้วยกระหม่อมฯ ข้าพระพุทธเจ้า บุคลากรศูนย์วิจัยคลินิก คณะแพทยศาสตร์ศิริราชพยาบาล

The 4th ARISE Annual Meeting in Nagasaki, Japan
SICRES participated in the 4th ARISE Annual Meeting held in Nagasaki, Japan

ATLAS–ARISE–PMDA International Symposium 2025
SICRES was honored to join this prestigious international symposium bringing together leaders in oncology, infectious disease, and regulatory science from across Asia.

Siriraj Welcomes the PDP Funders Group (PFG)
Siriraj had the great honor of welcoming the PDP Funders Group (PFG) — a global network of public and private funders supporting Product Development Partnerships (PDPs) to accelerate innovations in global health.
Who we are
Siriraj Institute of Clinical Research (SICRES) is an academic clinical research institute, operating under the Faculty of Medicine Siriraj Hospital, Mahidol University.
SICRES | ˈsīkres | , ไซ-เครส
"Your Success Is Our Success"
Our Mission
Our Vision
Our Goals
research program
Sustained collaboration with partners
Cutting edge research that advances clinical practice
Cost-effective services that bring value to our clients
Effective and timely communication of our findings
Our QUALITY POLICY
SICRES Management

Assoc. Prof. Dr. Pongsakorn Tantilipikorn, MD, PhD, FRCOT
Director of Siriraj Institute of Clinical Research
Pongsakorn Tantilipikorn, MD, PhD, is an Associate Professor of Rhinology and Allergy and serves as Chair of the Center of Research Excellence in Allergy and Immunology at the Faculty of Medicine Siriraj Hospital.
He currently serves as the Director of the Siriraj Institute of Clinical Research (SICRES), following many years of dedicated involvement and support in leadership roles, including Vice Director, where he played a key role in strengthening the institute’s clinical research capacity and strategic development. Dr. Pongsakorn received his Doctor of Medicine degree from Chiang Mai University in 1992, completed a postdoctoral fellowship in Rhinology at the University of Pennsylvania in 1999, and earned a PhD in Epidemiology and Biostatistics from Khon Kaen University in 2016.He is board certified in Otorhinolaryngology with subspecialty training in Facial Plastic and Reconstructive Surgery. His research interests include allergic rhinitis, rhinosinusitis, allergen immunotherapy, and endoscopic sinus surgery, along with advancing high-quality clinical research systems through his leadership at SICRES.

Prof. Kulkanya Chokephaibulkit, MD
Consultant & Founding Director
Kulkanya Chokephaibulkit is Professor of Pediatric Infectious Diseases and Founding Director of the Siriraj Institute of Clinical Research.

Prof. Kulkanya Chokephaibulkit, MD
Consultant & Founding Director
She earned the Doctor of Medicine from the Faculty of Medicine Siriraj Hospital, Mahidol University in 1986 and completed Pediatric residency in 1991.
Professor Chokephaibulkit is board certified by the American Academy of Pediatrics and Pediatric Infectious Diseases. Her research interests include HIV/AIDS in children, adolescents and pregnant women, antibiotics, vaccine in children, and tropical infectious diseases.

Assoc. Prof. Somruedee Chatsiricharoenkul, MD
Vice Director, Shared Services
Somruedee Chatsiricharoenkul is Associate Professor of Pharmacology and Vice Director of the Siriraj Institute of Clinical Research.

Assoc. Prof. Somruedee Chatsiricharoenkul, MD
Vice Director, Shared Services
She earned the Doctor of Medicine from the Faculty of Medicine Siriraj Hospital, Mahidol University in 2001 and completed residency training in Internal Medicine in 2005. In 2008, she earned a Diploma on Research and Product Development from Nagasaki-Thammasat University and a Certificate in Bioethics from the Asia Collaborative for Medical Education program in 2017.
Dr. Chatsirircharoenkul is the Secretary of the Siriraj Institutional Review Board and Assistant Dean for Research.

Assist. Prof. Dr. Suvimol Niyomnaitham, MD, MSCE, PhD
Vice Director, Clinical Research Support
Suvimol Niyomnaitham is Assistant Professor of Pharmacology and Vice Director of the Siriraj Institute of Clinical Research.

Assist. Prof. Dr. Suvimol Niyomnaitham, MD, MSCE, PhD
Vice Director, Clinical Research Support
She earned the Doctor of Medicine from the Faculty of Medicine at Siriraj Hospital, Mahidol University in 2006 and a Master’s Degree in Clinical Epidemiology and Biostatistics from the University of Pennsylvania in 2011.
In 2015, she earned a PhD in Pharmacoepidemiology at the University of Queensland with a focus on large database analysis. Now her research interest is cancer genomics. She also involves in clinical study designs and bioequivalence studies.

Mrs. Pornsuda Nipathakosol
Vice Director, Site Management Organization
Mrs. Pornsuda Nipathakosol is the Vice Director of the Siriraj Institute of Clinical Research

Mrs. Pornsuda Nipathakosol
Vice Director, Site Management Organization
What we do
SICRES conducts cost-effective clinical research at international standards and offers full-service clinical trial design and management. We offer a suite of capacities and services, including
- Experienced investigators and highly-trained clinical research staffs
- Precision clinical trial design and management (Phase I to IV)
- Bioequivalence studies, Feasibility surveys, Safety and Efficacy evaluations
- Integrated database development and biostatistical analysis
- Training and development of research staffs
- High impact medical publications and presentations
- Patient safety and adverse event monitoring
- A national and international research network
- Grant application support for investigators
Our Facilities
SICRES offers modern facilities that meet or surpass international standards. Our 30-bed research unit can accommodate overnight studies and has a synchronized clock system to assure protocol compliance. Each bed has a nurse call system, private restroom and 24-hour CCTV monitoring.
We have a comfortable patient waiting area, five examination rooms and a treatment room. SICRES also offers a secure conference room and private monitoring systems for investigators, sponsors and auditors. We maintain a secure filing and data archiving system to protect your data and assure patient confidentiality. We also operate separate fax systems for blinded and unblinded study staff. SICRES offers state of the art laboratory facilities including two 2-8°C, four sub 25°C refrigerators, one -20°C and three -80°C freezers.
All storage systems are maintained in 24-hour air-conditioned rooms with electronic remote notification of temperature variance. We have four refrigerated centrifuges, multiple biosafety cabinets and an emergency trolley equipped with a defibrillator and a 12-lead EKG.
บริการ
Clinical Research Services
SICRES offers a full spectrum of clinical research services to bring about new and innovative solution and findings. Our services cover the entire clinical research and development that can be tailored to national and international trials.

Feasibility Studies
Support the sponsors/CROs to evaluate the possibility of conducting a particular clinical trial at Faculty of Medicine, Siriraj Hospital or in a particular geographical region with the overall objective of optimum project completion in terms of timelines, targets and cost.

Study Planning and Budgeting
Supports our investigators/partners in the planning of their studies and budgeting processes by clearly understanding the collaborators’ requirements and offering customized project proposals.

Contract Management
Assist the sponsors/CROs by facilitating Clinical Trial Agreement (CTA) process.

Payment Management
Supports financial accountability on the basis of transparency, traceability and auditability.
Complete records of all financial transactions are maintained, making financial audits simple and easy.

Regulatory Submission
Support sponsors/CRO on preparation of clinical research dossiers for submission to Regulatory Authorities.
Assist investigators on the Institutional Review Board (IRB) / Independent Ethics Committee (IEC) submission.
Bioequivalence Center
30-bed clinical research facility well-equipped to conduct BE trials – whether on small molecule drugs or biosimilars – as well as phase 1 and early phase trials.
Supporting generic drug manufacturers in planning, initiating, and completing their BE trials fulfilling the regulatory requirements.

Clinic Facilities
Equipped with dedicated clinical research facilities for supporting ICH-GCP- standard clinical studies.
All such research facilities are properly maintained and are operated by professionally qualified and trained personnel.

Investigational Product Management
Support investigators in managing investigational products in compliance with Good Clinical Practice (GCP) requirements, with study drugs stored in strictly controlled units accessible only to authorized pharmacists, dispensers, and designated study personnel, and maintained in monitored refrigerators equipped with temperature data loggers, emergency power backup, uninterrupted power supply, and continuous 24/7 monitoring and alarm systems.

Sample Management
Equipped with regularly calibrated and well-maintained equipment to support biological specimen management services, including biosafety cabinets, refrigerated centrifuges, and incubators for specimen processing, with all medical freezers connected to 24-hour monitoring and alarm systems and supported by an emergency power supply.

Data Management
Provide highly professional, rapid, and exacting clinical data management services.
Our experienced staff assure the reliability of study’s data.

Statistical Analysis
Provide all-encompassing support, from consultation on the development of the statistical analysis plan to the creation of the statistical analysis report,
Perform statistical analyses to assess collected data objectively.

Medical Writing
Provide medical writing support for every stage in product development, from protocols, informed consent forms (ICF) to clinical study reports (CSR).
Clinical Operations
Our clinical operation services include: feasibility, site selection, contract negotiation and execution with clinical sites, clinical monitoring, supplying and retrieving investigational products, collection and checking case report forms, and processes for clinical studies closeouts.

Project Management
Provides skilled project management support for timely delivery of project milestones and performance targets for clinical trials.
Coordinate all aspects of the study closely with the sponsor and team.

Training and Education
Organizes various training programs for investigators, study coordinators, study nurses and other research staff to improve their capabilities to perform clinical trials.

Manuscript editing service
Polish a manuscript draft until it is ready to submit. Consultation service for writing medical articles is also provided.
Therapeutic Areas
Applied Thai Traditional Medicine
Dermatology
Allergy and Clinical Immunology
Cardiovascular
Endocrinology and Metabolism
Gastroenterology
Hematology
Infectious disease and tropical medicine
Oncology
Nephrology & Urology
Neurology
Respiratory Disease and Tuberculosis
Rheumatology
Obstetrics & Gynaecology
Ophthalmology
Orthopedics
Otorhinolaryngology
Pediatrics
Psychiatry
Vaccines
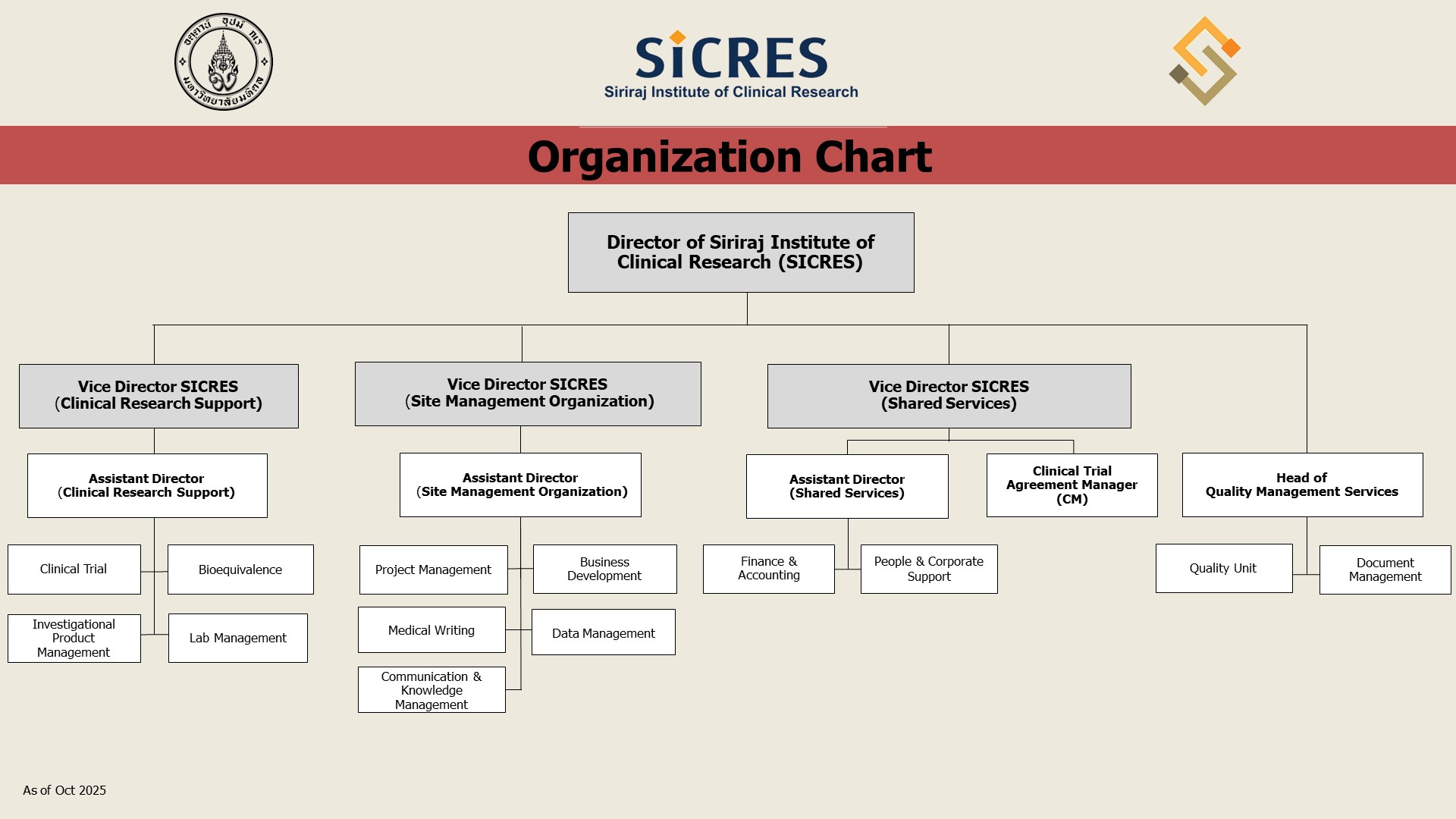
แผนผังองค์กร
- +66 2 412 8306 , +66 2 414 1899
- [email protected]
- ชั้น 10 ศูนย์วิจัยการแพทย์ศิริราช โรงพยาบาลศิริราช
- บางกอกน้อย กรุงเทพมหานคร ประเทศไทย
- ศูนย์วิจัยคลินิกคณะแพทยศาสตร์ศิริราชพยาบาล ขอสงวนลิขสิทธิ์ทุกประการ