> GET INVOLVED
Services
Clinical Research Services
SICRES offers a full spectrum of clinical research services to bring about new and innovative solution and findings. Our services cover the entire clinical research and development that can be tailored to national and international trials.

Feasibility Studies
Support the sponsors/CROs to evaluate the possibility of conducting a particular clinical trial at Faculty of Medicine, Siriraj Hospital or in a particular geographical region with the overall objective of optimum project completion in terms of timelines, targets and cost.

Study Planning and Budgeting
Supports our investigators/partners in the planning of their studies and budgeting processes by clearly understanding the collaborators’ requirements and offering customized project proposals.

Contract Management
Assist the sponsors/CROs by facilitating Clinical Trial Agreement (CTA) process.

Payment Management
Supports financial accountability on the basis of transparency, traceability and auditability.
Complete records of all financial transactions are maintained, making financial audits simple and easy.

IRB/IEC and Regulatory Submission
Support sponsors/CRO on preparation of clinical research dossiers for submission to Regulatory Authorities.
Assist investigators on the Institutional Review Board (IRB) / Independent Ethics Committee (IEC) submission.
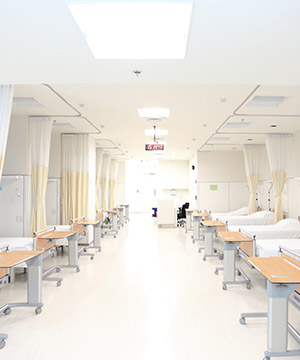
Bioequivalence Center
30-bed clinical research facility well-equipped to conduct BE trials – whether on small molecule drugs or biosimilars – as well as phase 1 and early phase trials.
Supporting generic drug manufacturers in planning, initiating, and completing their BE trials fulfilling the regulatory requirements.

Clinic Facilities
Equipped with dedicated clinical research facilities for supporting ICH-GCP- standard clinical studies.
All such research facilities are properly maintained and are operated by professionally qualified and trained personnel.

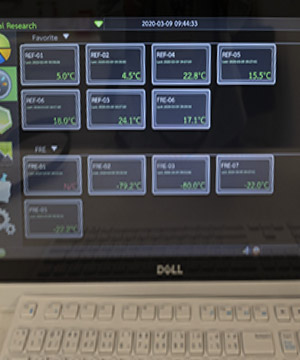
Investigational Product Management
Support investigator on the investigational product management in conformity with Good Clinical Practices (GCPs).
Strictly controlled units accessible only to authorized pharmacists, dispensers, and study drug management personnel.
All study drug refrigerators are monitored by temperature data loggers and supported by emergency electricity backup, uninterrupted power supply and round-the-clock monitoring and alarm system.

Sample Management
Equipped with regularly calibrated and properly maintained equipment for supporting biological specimen management services.
Biosafety cabinet, refrigerated centrifuges and incubator are available for processing of specimens. All medical freezers are connected to 24-hour monitoring and alarm systems and supported by emergency power supply.

Data Management
Provide highly professional, rapid, and exacting clinical data management services.
Our experienced staff assure the reliability of study’s data.

Statistical Analysis
Provide all-encompassing support, from consultation on the development of the statistical analysis plan to the creation of the statistical analysis report,
Perform statistical analyses to assess collected data objectively.

Medical Writing
Provide medical writing support for every stage in product development, from protocols, informed consent forms (ICF) to clinical study reports (CSR).
Clinical Operations
Our clinical operation services include: feasibility, site selection, contract negotiation and execution with clinical sites, clinical monitoring, supplying and retrieving investigational products, collection and checking case report forms, and processes for clinical studies closeouts.

Project Management
Provides skilled project management support for timely delivery of project milestones and performance targets for clinical trials.
Coordinate all aspects of the study closely with the sponsor and team.

Training and Education
Organizes various training programs for investigators, study coordinators, study nurses and other research staff to improve their capabilities to perform clinical trials.

Manuscript editing service
Polish a manuscript draft until it is ready to submit. Consultation service for writing medical articles is also provided.
Therapeutic Areas
Applied Thai Traditional Medicine
Dermatology
Allergy and Clinical Immunology
Cardiovascular
Endocrinology and Metabolism
Gastroenterology
Hematology
Infectious disease and tropical medicine
Oncology
Nephrology & Urology
Neurology
Respiratory Disease and Tuberculosis
Rheumatology
Obstetrics & Gynaecology
Ophthalmology
Orthopedics
Otorhinolaryngology
Pediatrics
Psychiatry
Vaccines
Investigators
Our investigators work across areas of medicines and play an essential role to healthcare development. By becoming one of SICRES investigators, you have opportunities to get involved in cutting-edge researches, to stay on top of the latest medicine development, and more importantly, to make better and healthier alternatives for your patients.
Assoc. Prof. TAWESAK TANWANDEE
อาสาสมัคร

การเป็นอาสาสมัครการวิจัยทางคลินิก
อาสาสมัครเป็นส่วนสำคัญที่ทำให้การวิจัยทางคลินิกสามารถพัฒนาให้เกิดความก้าวหน้าทางด้านสุขภาพ บุคคลใดก็ตามที่มีคุณสมบัติตรงตามเงื่อนไขของงานวิจัยอาจมีส่วนร่วมในการเข้าร่วมการวิจัยทางคลินิก
ก่อนเริ่มการพิจารณาในแต่ละครั้ง ทางศูนย์วิจัยทำงานร่วมกับคณะกรรมการจริยธรรมอิสระซึ่งประกอบด้วย นักวิชาชีพแพทย์ นักวิทยาศาสตร์ และนักวิจัย คณะกรรมการนี้กำหนดกระบวนการ ‘กระบวนการขอความยินยอม’ ซึ่งจะให้รายละเอียดของการศึกษาก่อนที่จะเข้าร่วมโครงการวิจัยทางคลินิก
นางสาวเกษศิรินทร์ ยะบุญวัน
นายภาสกร กาญจนโอฬาร
กระบวนการขอความยินยอม
การวิจัยทางคลินิกจะต้องดำเนินการภายใต้การกำกับดูแลของแพทย์ซึ่งเป็นผู้เชี่ยวชาญในสาขาการวิจัยที่เกี่ยวข้องซึ่งเรียกว่านักวิจัยหลัก ต้องแจ้งข้อมูลที่ชัดเจนให้กับกับอาสาสมัคร ได้แก่
- วัตถุประสงค์การศึกษา
- ระเบียบการวิจัย
- ประโยชน์ที่คาดหวัง
- ภาระผูกพันและความเสี่ยงที่คาดการณ์ได้
- สิทธิ์ในการปฏิเสธที่จะเข้าร่วมในการศึกษา
- เอกสารที่ได้รับความยินยอมเป็นลายลักษณ์อักษรตามกฎหมาย
อาสาสมัครผู้ใดก็ตามที่รับความยินยอมให้เข้าร่วมในการศึกษาสามารถที่จะถอนความยินยอมได้ทุกเมื่อและสิ้นสุดการเข้าร่วมในการศึกษาโดยไม่มีผลกระทบใด ๆ ต่อการดูแลรักษาในอนาคต
สนใจเป็นอาสาสมัครการวิจัยทางคลินิก?
หากท่านต้องการมีส่วนร่วมในการวิจัยทางคลินิก
สามารถติดต่อมาได้ที่ ศูนย์วิจัยคลินิก คณะแพทยศาสตร์ศิริราชพยาบาล เบอร์ติดต่อ 02-414-1899 หรือ 02-412-8306
Partners
- ClinActis
- Global Antibiotic Research and Development Partnership (GARDP)
- Government Pharmaceutical Organization (GPO)
- GreenLight
- ICON Clinical Research
- IQVIA
- Novotech
- Oregon Health & Science University (OHSU)
- Parexel
- PPD
- PRA health sciences
- The Drugs for Neglected Diseases initiative (DNDi)
- The National Center for Global Health and Medicine (NCGM)
- University of California, Los Angeles (UCLA)
Dr. Sasi Suda
Founder & CEO, GreenLight Clinical
Testimonial

Jean-Michel Piedagnel
Director - South-East Asia
Drugs For Neglected Diseases Initiative
o Could you please introduce yourself
I am the Director of Regional Office, DNDi South-East Asia and joined DNDi as Head of the South-East Asia office, in April 2016 after more than 20 years in the medical non-for-profit sector
o Describe your experience with SICRES (and/or Siriraj CRC)
Our relationship with SICRES is recent but I feel there is a real alignment of values between the two organisations. I also enjoy their professionalism and expertise in clinical trials.
o What are major project outcomes of your projects e.g. social impact and impacts on patients?
The core focus of our organisation, DNDi is access to treatments for all patients, including those who are neglected. As a not-for-profit organisation, it is important and crucial for us that the treatments we put on the markets are affordable and accessible to everyone in need of treatment, especially neglected patients, so that no one gets left behind. We share that ambition with SICRES.
o Will you want to have more projects and work with SICRES in the future?
I hope we can develop a strategic partnership with SICRES over the years to come in medical research
o What make SICRES better than other clinical research institution?
(Why SICRES not the others?)
It is a non for profit value driven organization with in depth expertise and professionalism on medical research
o Why do you trust SICRES? Why do you choose to work with SICRES?
It is a non for profit value driven organization with in depth expertise and professionalism on medical research
o Please describe how SICRES can help you reach your expected outcome? (Impressive work of SICRES)
SICRES has an impressive track record in clinical trials and with their new ambitions and leadership, I see they can offer the same services as a CRO but in a non for profit environment and this is exactly what is needed currently in Thailand. Thailand as a higher middle-income country can become a world leading actor of medical R&D. This is part of DNDi mandate to encourage such development. SICRES is exactly the type of organization that Thailand need to build on that potential.
o Please describe SICRES in your view
It is a not-for-profit value driven organization with in-depth expertise and professionalism on medical research. They offer cost effective clinical research at international standards and offers full-service clinical trial design and management. At the same time, they also offer modern facilities that meets international standards requirements for the purpose of clinical research/trials
Sponsors/Funders
Sponsors
- ALexion Pharmaceutical Inc.
- Alivio Therapeutics
- ALLERGAN THAILAND, LLC
- AnaptysBio Inc.
- Apexcela Co. Ltd.
- Ascendant Biotech Corporation
- Asklepios Biopharmaceuticals, Inc.
- Astellas Pharma Inc.
- AstraZeneca (Thailand) Ltd.
- Auris Medical AG
- Bayer Thai Company Limited
- Beiersdorf (Thailand) Co., Ltd.
- BLACKMORES LIMITED
- Boehringer Ingelheim Singapore
- Canadian/Toronto based HV study
- Checkpoint Therapeutics, Inc.
- Cidara Therapeutics, Inc
- Cipla Ltd
- Cubist Pharmaceuticals
- Dutch Mill Co., Ltd.
- EU Biotech
- F2G Biotech GmbH
- Ferrokin
- F-Hoffman La Roche (Thailand) Ltd.
- GlaxoSmithKline (Thailand) Limited
- Great Eastern Drug Co., Ltd
- Inovio Pharmaceuticals, Inc.
- Health Systems Research Institute
- Insmed Incoporated
- Instylla Inc.
- Janssen-Cilag Ltd.,
- LG Life Sciences Ltd.
- Medi Us Co.,Ltd.
- MERCK & Co Inc
- Millennium Pharmaceuticals Inc.
- MSD (THAILAND) LTD.,
- National Center for Global Health and Medicine (NCGM)
- Nodthera
- Novartis (Thailand) Ltd.
- PCI Biotech
- Pfizer Thailand Ltd.
- Pharmaland (1982) Co.,Ltd.
- PT Kalbe Genexine Biologics
- Puma Biotechnology
- Rajburi Sugar Co., Ltd.
- Regeneron Pharmaceuticals, Inc.
- Ripple Therapeutics Corporation
- Roche (Thailand) Ltd.
- S.C. Artistry Co., Ltd.
- Sideris Pharmaceuticals, Inc
- Sillajen Inc.
- TOLMAR IInc.
- UCB Japan Co. Ltd.
- UK based biotech
Donors
คุณ ดวงพร เที่ยงวัฒนธรรม
รองกรรมการผู้จัดการใหญ่ กำกับดูแลองค์กรและกิจการสัมพันธ์ ปตท.
Your support make a valuable impact.
Your donation helps improve lives and better health in the community. Despite the increasing technology and innovation made towards facilitating health in today’s society, there are more pressing needs in clinical research than ever. Therefore, your support will be fueling vital research that seeks solutions and answers to challenging and complex research contexts.
Donate Now
สนใจบริจาคเข้าบัญชีสะสมทรัพย์
ธนาคารกรุงเทพ
สาขารพ.ศิริราช ปิยมหาราชการุณย์
เลขบัญชี 901-7-06257-2
ชื่อบัญชี ศิริราชมูลนิธิ ทุนวิจัยเพื่อผู้ป่วย D004015

