> RESEARCH & SUPPORT
Services
Clinical Research Services
SICRES offers a full spectrum of clinical research services to bring about new and innovative solution and findings. Our services cover the entire clinical research and development that can be tailored to national and international trials.

Feasibility Studies
Support the sponsors/CROs to evaluate the possibility of conducting a particular clinical trial at Faculty of Medicine, Siriraj Hospital or in a particular geographical region with the overall objective of optimum project completion in terms of timelines, targets and cost.

Study Planning and Budgeting
Supports our investigators/partners in the planning of their studies and budgeting processes by clearly understanding the collaborators’ requirements and offering customized project proposals.

Contract Management
Assist the sponsors/CROs by facilitating Clinical Trial Agreement (CTA) process.

Payment Management
Supports financial accountability on the basis of transparency, traceability and auditability.
Complete records of all financial transactions are maintained, making financial audits simple and easy.

IRB/IEC and Regulatory Submission
Support sponsors/CRO on preparation of clinical research dossiers for submission to Regulatory Authorities.
Assist investigators on the Institutional Review Board (IRB) / Independent Ethics Committee (IEC) submission.
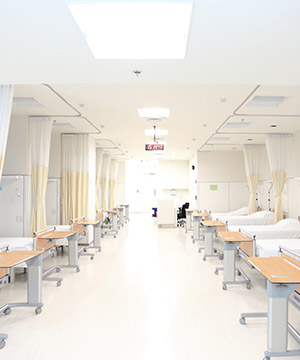
Bioequivalence Center
30-bed clinical research facility well-equipped to conduct BE trials – whether on small molecule drugs or biosimilars – as well as phase 1 and early phase trials.
Supporting generic drug manufacturers in planning, initiating, and completing their BE trials fulfilling the regulatory requirements.

Clinic Facilities
Equipped with dedicated clinical research facilities for supporting ICH-GCP- standard clinical studies.
All such research facilities are properly maintained and are operated by professionally qualified and trained personnel.

Investigational Product Management
Support investigator on the investigational product management in conformity with Good Clinical Practices (GCPs).
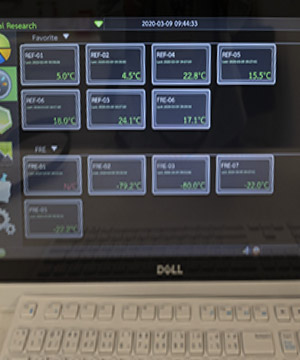
Strictly controlled units accessible only to authorized pharmacists, dispensers, and study drug management personnel.
All study drug refrigerators are monitored by temperature data loggers and supported by emergency electricity backup, uninterrupted power supply and round-the-clock monitoring and alarm system.

Sample Management
Equipped with regularly calibrated and properly maintained equipment for supporting biological specimen management services.
Biosafety cabinet, refrigerated centrifuges and incubator are available for processing of specimens. All medical freezers are connected to 24-hour monitoring and alarm systems and supported by emergency power supply.

Data Management
Provide highly professional, rapid, and exacting clinical data management services.
Our experienced staff assure the reliability of study’s data.

Statistical Analysis
Provide all-encompassing support, from consultation on the development of the statistical analysis plan to the creation of the statistical analysis report,
Perform statistical analyses to assess collected data objectively.

Medical Writing
Provide medical writing support for every stage in product development, from protocols, informed consent forms (ICF) to clinical study reports (CSR).
Clinical Operations
Our clinical operation services include: feasibility, site selection, contract negotiation and execution with clinical sites, clinical monitoring, supplying and retrieving investigational products, collection and checking case report forms, and processes for clinical studies closeouts.

Project Management
Provides skilled project management support for timely delivery of project milestones and performance targets for clinical trials.
Coordinate all aspects of the study closely with the sponsor and team.

Training and Education
Organizes various training programs for investigators, study coordinators, study nurses and other research staff to improve their capabilities to perform clinical trials.

Manuscript editing service
Polish a manuscript draft until it is ready to submit. Consultation service for writing medical articles is also provided.
Therapeutic Areas
Applied Thai Traditional Medicine
Dermatology
Allergy and Clinical Immunology
Cardiovascular
Endocrinology and Metabolism
Gastroenterology
Hematology
Infectious disease and tropical medicine
Oncology
Nephrology & Urology
Neurology
Respiratory Disease and Tuberculosis
Rheumatology
Obstetrics & Gynaecology
Ophthalmology
Orthopedics
Otorhinolaryngology
Pediatrics
Psychiatry
Vaccines
Otorhinolaryngology
Pediatrics
Psychiatry
Useful Links
Process Flowcharts
Flow for CTA : Link > https://www.si.mahidol.ac.th/th/research-academics/research/test_research_fund_com.asp
Siriraj Institutional Review Board (IRB)
Training & Development

Announcement of Eligible Participants for the Clinical Vaccine Trial Training Program!
ประกาศรายชื่อ ผู้มีสิทธิ์เข้าอบรม “หลักสูตรการวิจัยวัคซีนทางคลินิก สำหรับแพทย์ เภสัชกรณ์ และพยาบาลวิจัย” Clinical Vaccine Trial Expert Program!

Register now! Clinical Vaccine Trial Training
เปิดรับสมัครแล้ววันนี้! สำหรับโครงการอบรมฟรี “หลักสูตรการวิจัยวัคซีนทางคลินิก สำหรับแพทย์ เภสัชกรณ์ และพยาบาลวิจัย”

Download now! ICH GCP R3 Certificate
Ready to download Now! ICH GCP R3 (Good Clinical Practice) Training Certificate organized by Siriraj Institute of Clinical Research

Register now! “ICH GCP R3” on May 30 2025 Onsite only
ศูนย์วิจัยคลินิก SICRES เปิดรับสมัครผู้เข้าร่วมอบรมหลักสูตร ICH GCP R3 เฉพาะบุคลากรภายในคณะแพทยศาสตร์ศิริราชพยาบาลเท่านั้น

PPD Visit and Cell & Gene Therapy Training
SICRES is honored to welcome PPD for an insightful visit and training session on Cell & Gene Therapy.

SICRES CPR Training 2023
ศูนย์วิจัยคลินิก คณะแพทยศาสตร์ศิริราชพยาบาล SICRES จัดกิจกรรม CPR Mock-up ประจำปี 2566
project Archives
A list of project archives
SICRES has in place a Standard Operating Procedures (SOP) consistent with Good Clinical Practice (GCP) Guideline, Regulatory Authority Requirements, and Human Research Protection Unit Policy and Guideline.
Previous studies on Bioequivalence (BE)
1. Amlodipine tablet 10 mg
2. Atorvastatin tablet 40mg
3. Bambuterol tablet 10 mg
4. Carvedilol tablet 25 mg
5. Cefoperazone/Sulbactam 1/0.5 g IM
6. Cilostazol tablet 50 mg
7. Clopidogrel tablet 75 mg
8. Donepezil tablet 10 mg
9. Glimepiride tablet 3 mg
10. Glucosamine capsule 500 mg
11. Glucosamine sachet 1500 mg
12. Irbesartan tablet 300mg
13. Levothyroxine Sodium
14. Losartan tablet 100 mg
15. Losartan/HCTZ tablet 100/25 mg
16. Manidipine tablet 20 mg
17. Montelukast tablet 10 mg
18. Olanzapine tablet 10 mg
19. Oseltamivir tablet 75 mg
20. Pioglitazone tablet 30mg
21. Ramipril tablet 10 mg
22. Sertraline tablet 50 mg
23. Spironolactone tablet 100 mg
24. Tamsulosin ER capsule 0.2 mg
25. Tenofovir tablet 300 mg
26. Trimetazidine MR 35 mg
27. Valsartan tablet 160 mg
28. Venlafaxine XR capsule 75 mg